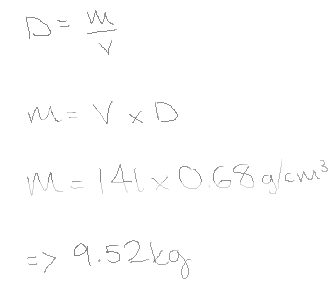(56)What is the mass, in kilograms, of 14.0 L of gasoline? (Assume that the density of gasoline is 0.680 g/cm3.)
9.52kg
Thats the question, and answer, from a recent quiz I took in school today.
All I understand in Density = Mass/ Volume.
I am having serious issues doing the whole inverse operations with problems like these gonk If anyone is willing to sit through my questions, please help!
Note: In general, I understand inverse operations. + becomes - , / becomes x. So on. But with chemistry, my brain just dies. neutral
For those of you who took Chemistry or what you consider your most challenging course... How did you get through the days where nothing seemed to click?
It's A Girl Thing! ♥
A Family, A Home.
 |
|
|||||
|
||||||
|